Anaphylactic Biosensor and Monitor
November 2022 - December 2023 • Cornell University • BME 1310: Introduction to Biomedical Engineering • Product Design, Research and Development, and Applications of Bioengineering
For the final project for my Introduction to Biomedical Engineering class, myself and a team of biomedical engineers identified a market gap for allergy maintenance technology in the medical community and proposed a design of a potential continuous monitoring solution for individuals at risk for anaphylactic attacks. I was involved in leading the technical design process of the device and particularly synthesized a device that would quantify various factors associated with severe anaphylaxis (histamines, bradykinin, and platelet-activating factor) to assist patients in anticipating and managing the onset of an allergy episode. Although the project was a group effort, I specifically and solely provided contributions towards the executive summary, the design criteria, the engineering process, manufacturing, and diagrams within the pdf provided below. The following is an overview of my contributions to the designing, process engineering, and technical principles.
Problem Statement
In the United States, upwards of 5% of the population has suffered a severe anaphylactic attack, and additionally, nearly half have required medical intervention, including self-administration of drugs. While auto-injector technology prevails as the dominant treatment, rapid increases in market prices of common auto-injectors such as EpiPen have made the practice of self-administered auto-injectors more economically impractical on top of stressful. The Anaphylactic BioSensor and Monitor (ABSM) would be a patch similar to a glucose patch that monitors multiple anaphylaxis-specific proteins released within a patient, thus creating a more specialized and effective device in detecting specifically type I hypersensitivity.
Ideation
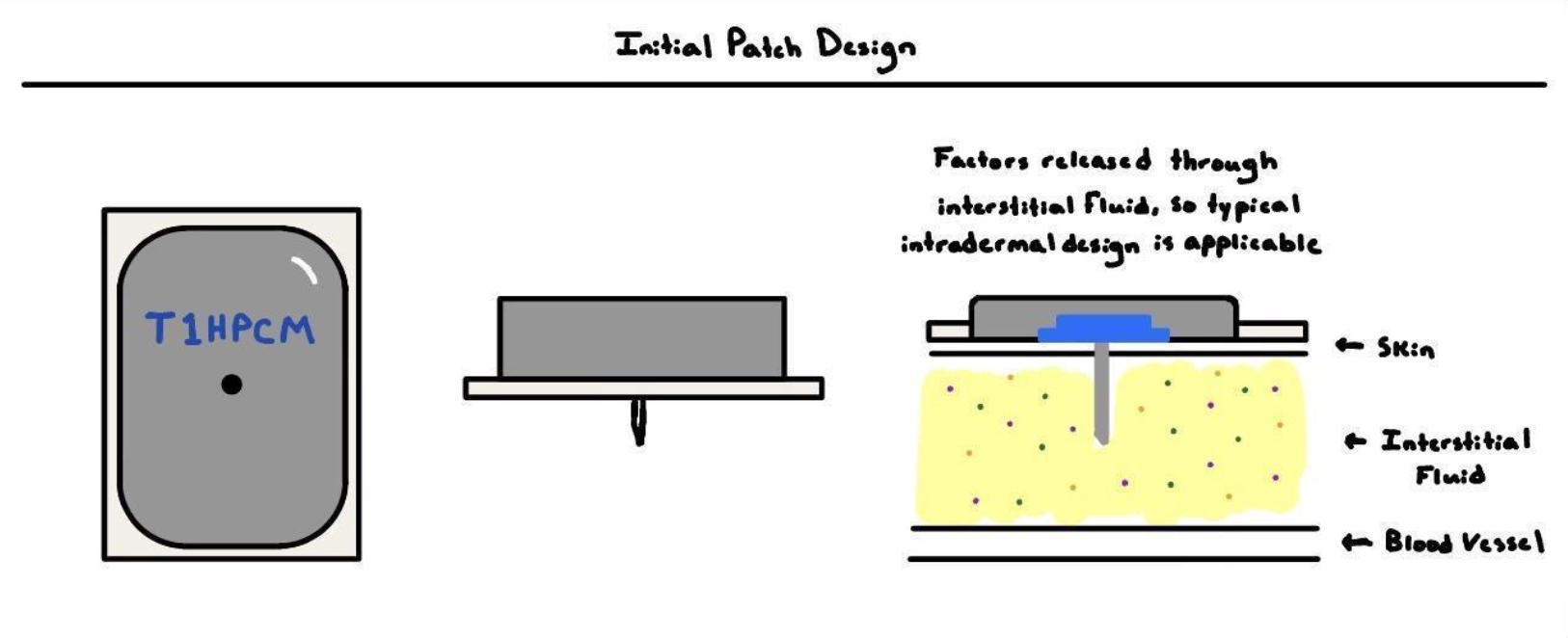
The device is intended to constantly monitor the concentration of analytes in the blood, namely histamines, platelet-activating factors, and bradykinin, to indicate oncoming anaphylaxis. More specifically, the device determines possess bioreceptors that analyze fluids containing anaphylaxis-associated proteins and calculate the rate of analyte production.
In terms of design and patient application, the device is inspired by modern-day glucose monitors, which have similar design requirements such as electronics, ease of application, comfortability, etc. First, the ABSM quantifies the presence of certain analytes in the body through bioreceptors that induce a specific current on the probe, which is extrapolated for the rate of analyte production. Additionally, the device is administered using a spring-loaded mechanism similar to continuous glucose monitors such as the Abbott Freestyle Libre. Finally, the device is intended to be replaced every month for both sanitation and efficacy. Overall, the design is an adaptation.
Manufacturing
Starting from constructing the smallest piece of the device, the sensors within the needle, a private, in-house biomedical laboratory is a necessity with tools such as a Horizontal Silicon Wafer Wet Etching and Washing Machine. However, certain aspects are able to be outsourced to other manufacturing companies such as the production of PCBs for the patch, reducing the equipment and skill necessary for a manufacturing facility for the patch. Additionally, some simple machinery would be needed for binding the needle receptor to the electronic board, securing the electronic board within the plastic casing, and attaching the adhesive patch to the base of the unit. Overall, the building would comprise two spaces, one for bioengineering the sensors and another for assembling the final patch, indicating the necessity for a building of about 100,000 square feet for the entire operation.
Engineering Process
The module is composed of three major components, including the needle receptor, the transmitter, and the supplementary application. The bioreceptor encased in the needle protrudes 0.25 cm from the center point of the base of the enter module, representing the total length the device will be implanted in the patient's skin. The required location is on the lower abdomen above the waistline since the device is orientation sensitive (based particularly on gravity) and the torso changes rotational orientation compared to other conventional patch locations such as the arm or thigh. Novel affinity-based piezoelectric microcantilever sensors are implemented as the primary sensors of the device, utilizing compressive forces created by antibody binding to create an electrical current that can be measured and analyzed. These sensors are produced through the micro-electromechanical systems (MEMS) technique, one that is repeated for each analyte analyzed by the device. An additional process is necessary to attach the antibody receptors, and a process of direct coupling is utilized to bind antibodies to the surface of the sensor. Finally, signals are amplified and filtered on the device, and data is relayed from the device to a user app for patients to interact with.